

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50450653 (CHEMBL2115493) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Bioorganic Research Curated by ChEMBL | Assay Description Inhibitory activity against forskolin-stimulated cAMP production in chinese hamster ovary cells expressing Opioid receptor delta 1 | Bioorg Med Chem Lett 8: 2027-32 (1999) BindingDB Entry DOI: 10.7270/Q25T3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50450653 (CHEMBL2115493) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Bioorganic Research Curated by ChEMBL | Assay Description Inhibitory activity against forskolin-stimulated cAMP production in chinese hamster ovary cells expressing Opioid receptor delta 1 | Bioorg Med Chem Lett 8: 2027-32 (1999) BindingDB Entry DOI: 10.7270/Q25T3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50450656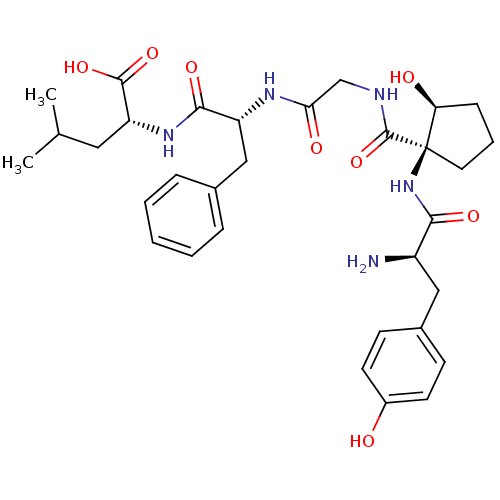 (CHEMBL2114469) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Bioorganic Research Curated by ChEMBL | Assay Description Inhibitory activity against forskolin-stimulated cAMP production in chinese hamster ovary cells expressing Opioid receptor delta 1 | Bioorg Med Chem Lett 8: 2027-32 (1999) BindingDB Entry DOI: 10.7270/Q25T3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50228042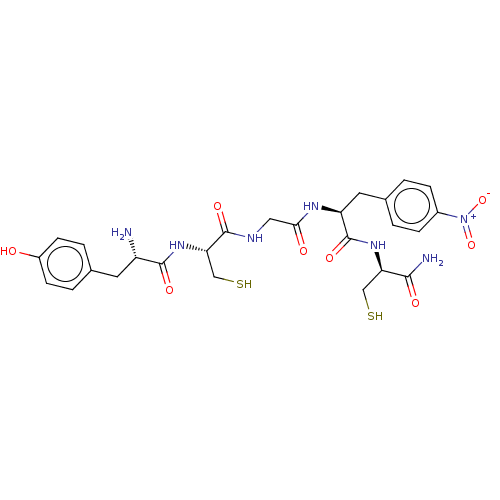 (CHEMBL323548) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0187 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace delta-receptor specific radioligand [3H]DPDPE | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50450656 (CHEMBL2114469) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Bioorganic Research Curated by ChEMBL | Assay Description Binding affinity against rat Opioid receptor delta 1 expressed in CHO cells determined by competitive inhibition of 1 nM [3H]DPDPE. | Bioorg Med Chem Lett 8: 2027-32 (1999) BindingDB Entry DOI: 10.7270/Q25T3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM144637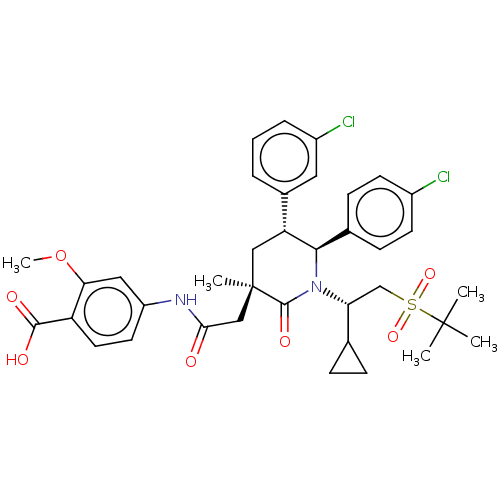 (US8952036, Ex. 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0503 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071201 ((R)-2-[(R)-2-(2-{(R)-2-[(R)-2-Amino-3-(4-hydroxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Bioorganic Research Curated by ChEMBL | Assay Description Inhibitory activity against forskolin-stimulated cAMP production in chinese hamster ovary cells expressing Opioid receptor delta 1 | Bioorg Med Chem Lett 8: 2027-32 (1999) BindingDB Entry DOI: 10.7270/Q25T3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50448963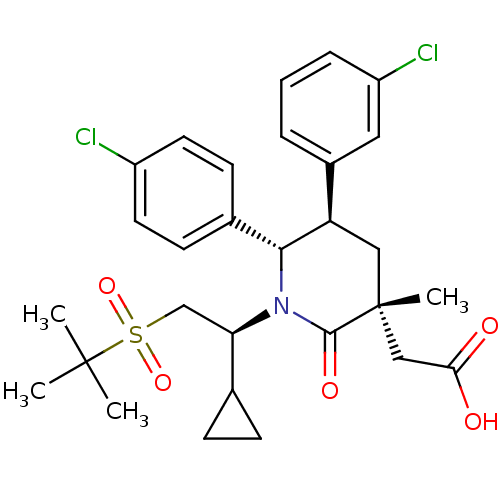 (CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0962 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50337733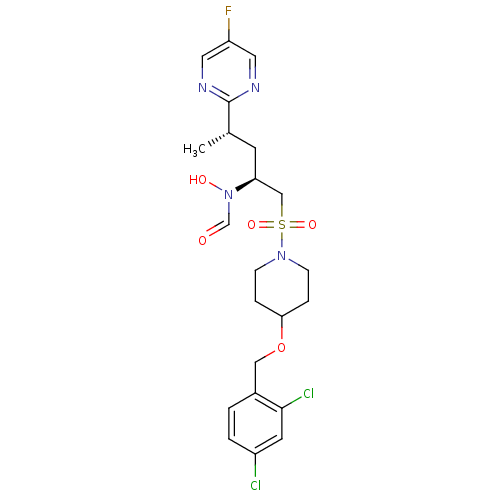 (CHEMBL1683444 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | Bioorg Med Chem Lett 21: 1376-81 (2011) Article DOI: 10.1016/j.bmcl.2011.01.036 BindingDB Entry DOI: 10.7270/Q2DV1K5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM144636 (US8952036, Ex. 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Eur J Med Chem 101: 627-39 (2015) Article DOI: 10.1016/j.ejmech.2015.06.029 BindingDB Entry DOI: 10.7270/Q2QF8VPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50061291 ((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain | J Med Chem 40: 3957-62 (1998) Article DOI: 10.1021/jm9704762 BindingDB Entry DOI: 10.7270/Q23R0TJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50330011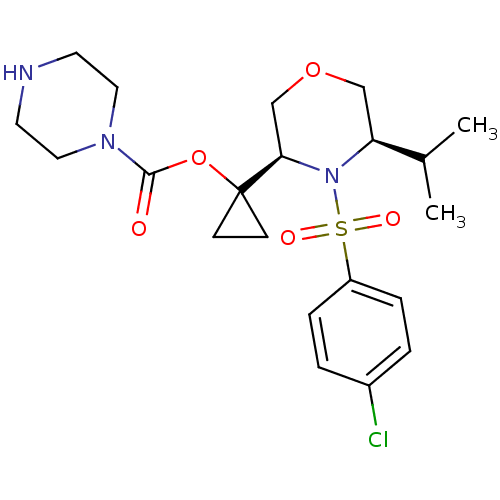 (1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 after 30 mins | Bioorg Med Chem Lett 20: 6606-9 (2010) Article DOI: 10.1016/j.bmcl.2010.09.028 BindingDB Entry DOI: 10.7270/Q2RF5V79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039026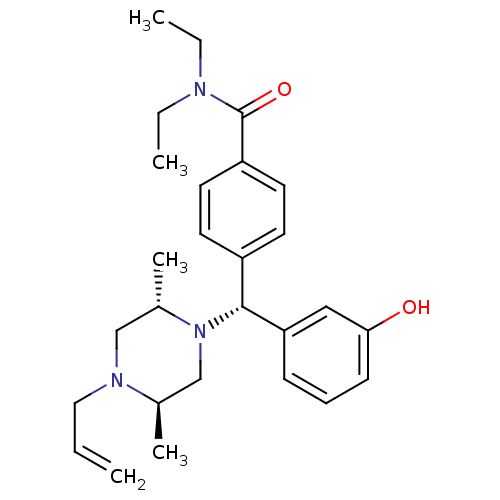 (4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona Curated by ChEMBL | Assay Description Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. | J Med Chem 41: 4767-76 (1998) Article DOI: 10.1021/jm980374r BindingDB Entry DOI: 10.7270/Q2DZ07FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM144638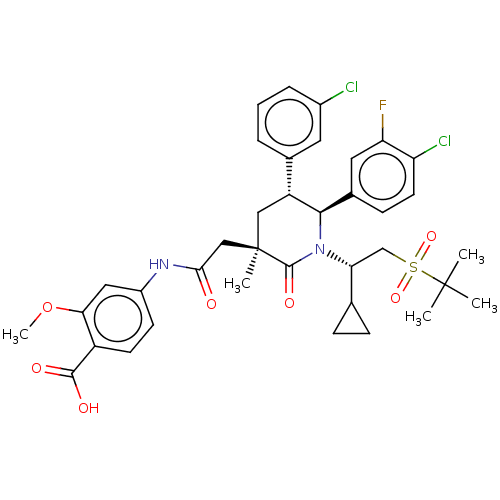 (US8952036, Ex. 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen Inc. US Patent | Assay Description The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... | US Patent US8952036 (2015) BindingDB Entry DOI: 10.7270/Q27P8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50037079 (CHEMBL3355781) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H][Ile5,6]deltorphin-2 from DOR in Wistar rat brain membranes by liquid scintillation counting method | Bioorg Med Chem 22: 6545-51 (2015) Article DOI: 10.1016/j.bmc.2014.10.022 BindingDB Entry DOI: 10.7270/Q2HT2QZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM143355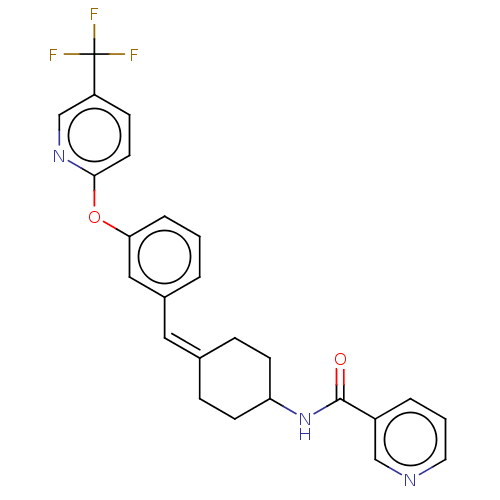 (US9682953, 20.A-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 5 mins followed by NADPH cofactor addition and measur... | Bioorg Med Chem Lett 29: 238-243 (2019) Article DOI: 10.1016/j.bmcl.2018.11.048 BindingDB Entry DOI: 10.7270/Q2125X14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071201 ((R)-2-[(R)-2-(2-{(R)-2-[(R)-2-Amino-3-(4-hydroxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Bioorganic Research Curated by ChEMBL | Assay Description Binding affinity against rat Opioid receptor delta 1 expressed in CHO cells determined by competitive inhibition of 1 nM [3H]DPDPE. | Bioorg Med Chem Lett 8: 2027-32 (1999) BindingDB Entry DOI: 10.7270/Q25T3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50061292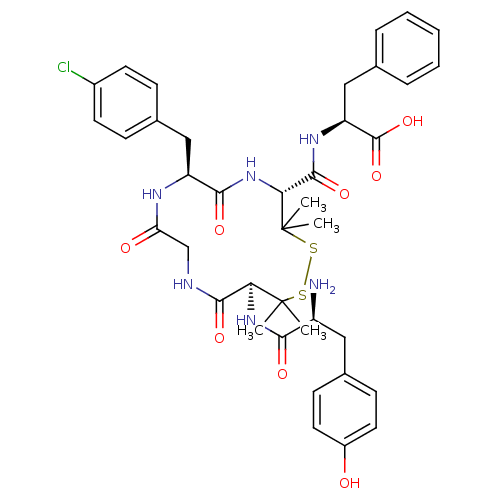 ((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain | J Med Chem 40: 3957-62 (1998) Article DOI: 10.1021/jm9704762 BindingDB Entry DOI: 10.7270/Q23R0TJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50252094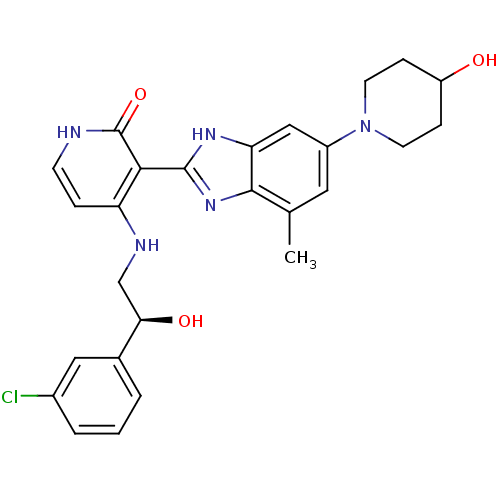 ((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4075-80 (2008) Article DOI: 10.1016/j.bmcl.2008.05.104 BindingDB Entry DOI: 10.7270/Q2V40V03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50061295 ((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain | J Med Chem 40: 3957-62 (1998) Article DOI: 10.1021/jm9704762 BindingDB Entry DOI: 10.7270/Q23R0TJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50061293 ((S)-2-{[(4R,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of binding of radioligand [3H][p-Cl-phe]-DPDPE to Opioid receptor delta 1 in rat brain | J Med Chem 40: 3957-62 (1998) Article DOI: 10.1021/jm9704762 BindingDB Entry DOI: 10.7270/Q23R0TJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50009180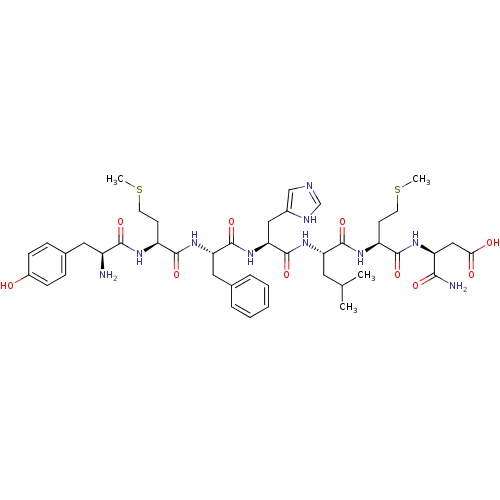 (3-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of [3H]-[p-Cl-Phe-]DPDE binding to rat brain homogenate delta-opioid receptor | J Med Chem 37: 141-5 (1994) BindingDB Entry DOI: 10.7270/Q2F47N7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM27879 (4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4075-80 (2008) Article DOI: 10.1016/j.bmcl.2008.05.104 BindingDB Entry DOI: 10.7270/Q2V40V03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50330016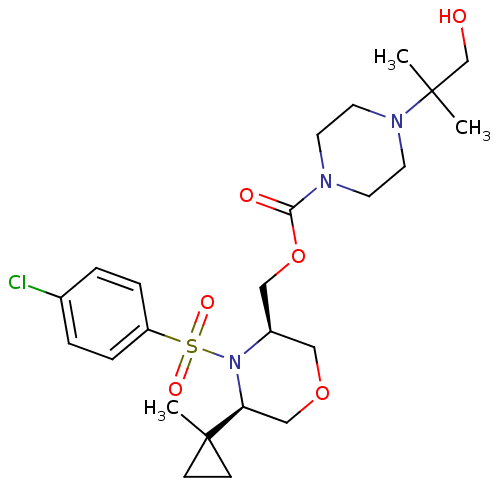 (((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(1-methylcyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 after 30 mins | Bioorg Med Chem Lett 20: 6606-9 (2010) Article DOI: 10.1016/j.bmcl.2010.09.028 BindingDB Entry DOI: 10.7270/Q2RF5V79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50252295 (3-(6-(4-((1R,4S)-5-oxa-2-aza-bicyclo[2.2.1]heptan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4075-80 (2008) Article DOI: 10.1016/j.bmcl.2008.05.104 BindingDB Entry DOI: 10.7270/Q2V40V03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50252237 ((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4075-80 (2008) Article DOI: 10.1016/j.bmcl.2008.05.104 BindingDB Entry DOI: 10.7270/Q2V40V03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50330006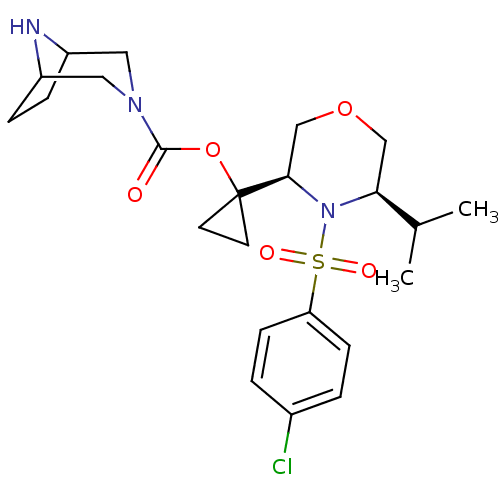 (1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 after 30 mins | Bioorg Med Chem Lett 20: 6606-9 (2010) Article DOI: 10.1016/j.bmcl.2010.09.028 BindingDB Entry DOI: 10.7270/Q2RF5V79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50043720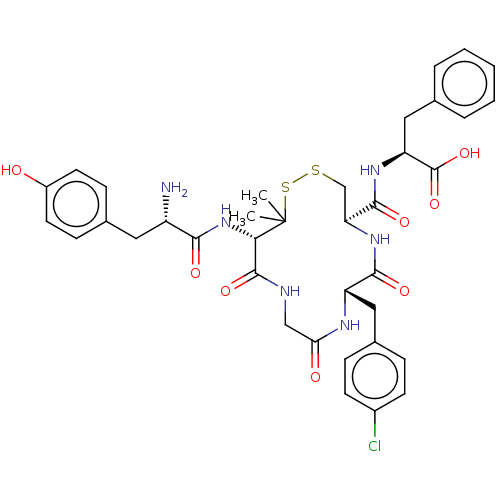 ((S)-2-{[(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand | J Med Chem 37: 146-50 (1994) BindingDB Entry DOI: 10.7270/Q2TX3G09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468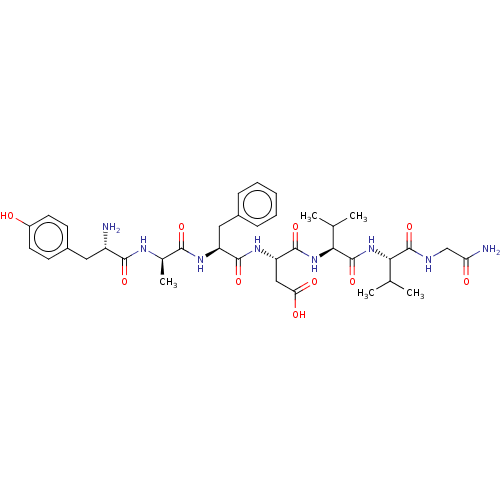 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Agonist activity for Opioid receptor delta 1 | J Med Chem 40: 3100-8 (1997) Article DOI: 10.1021/jm9607663 BindingDB Entry DOI: 10.7270/Q2TD9Z1N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from delta opioid receptor in rat brain membranes | Bioorg Med Chem Lett 18: 1350-3 (2008) Article DOI: 10.1016/j.bmcl.2008.01.009 BindingDB Entry DOI: 10.7270/Q2QR4WWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against delta receptor with [3H]-[p-Cl-Phe4]-DPDPE | Bioorg Med Chem Lett 2: 547-552 (1992) Article DOI: 10.1016/S0960-894X(01)81195-1 BindingDB Entry DOI: 10.7270/Q23R0STD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50007329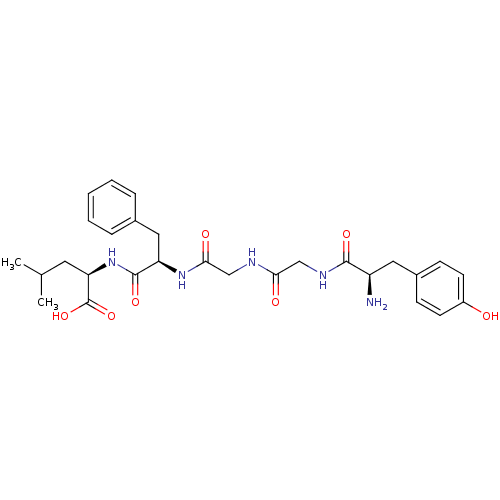 ((R)-2-[(R)-2-(2-{2-[(R)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Bioorganic Research Curated by ChEMBL | Assay Description Binding affinity against rat Opioid receptor delta 1 expressed in CHO cells determined by competitive inhibition of 1 nM [3H]DPDPE. | Bioorg Med Chem Lett 8: 2027-32 (1999) BindingDB Entry DOI: 10.7270/Q25T3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Bioorganic Research Curated by ChEMBL | Assay Description Binding affinity against rat Opioid receptor delta 1 expressed in CHO cells determined by competitive inhibition of 1 nM [3H]DPDPE. | Bioorg Med Chem Lett 8: 2027-32 (1999) BindingDB Entry DOI: 10.7270/Q25T3KZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50252193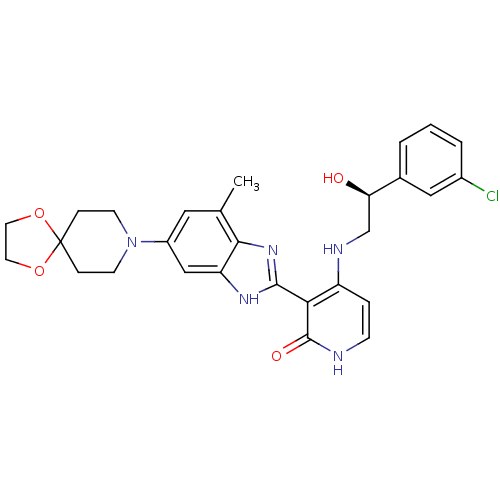 (4-((S)-2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4075-80 (2008) Article DOI: 10.1016/j.bmcl.2008.05.104 BindingDB Entry DOI: 10.7270/Q2V40V03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50252236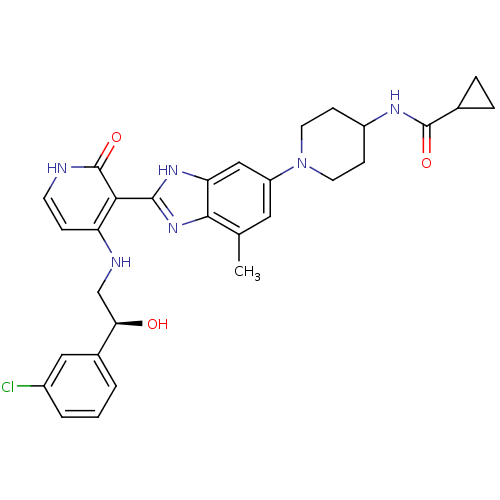 ((S)-N-(1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4075-80 (2008) Article DOI: 10.1016/j.bmcl.2008.05.104 BindingDB Entry DOI: 10.7270/Q2V40V03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50043725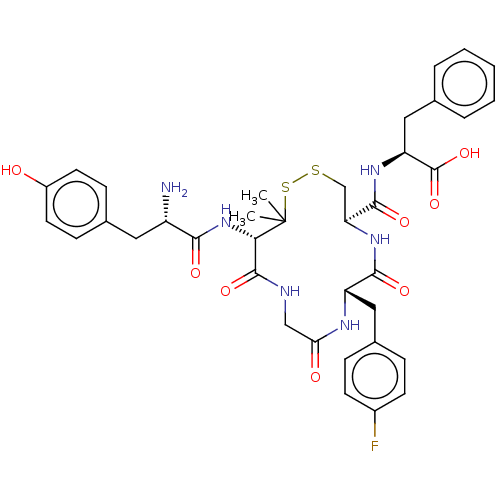 ((S)-2-{[(4R,7S)-13-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Binding affinity towards delta opioid receptor was determined in rat brain using [H]-[p-Cl-Phe4]-DPDPE as radioligand | J Med Chem 37: 146-50 (1994) BindingDB Entry DOI: 10.7270/Q2TX3G09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of [3H]-[p-Cl-Phe-]DPDE binding to rat brain homogenate delta-opioid receptor | J Med Chem 37: 141-5 (1994) BindingDB Entry DOI: 10.7270/Q2F47N7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of [3H]-[p-Cl-Phe-]DPDE binding to rat brain homogenate delta-opioid receptor | J Med Chem 37: 141-5 (1994) BindingDB Entry DOI: 10.7270/Q2F47N7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50330017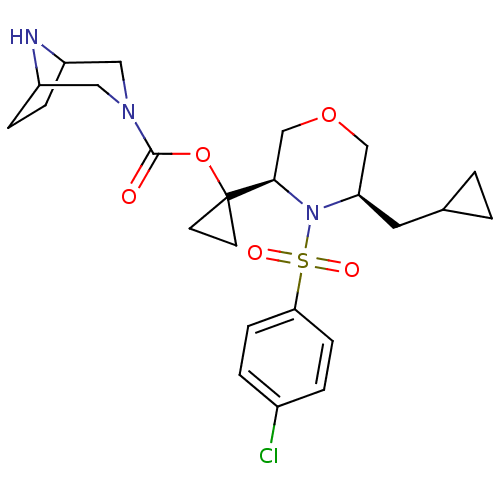 (1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 after 30 mins | Bioorg Med Chem Lett 20: 6606-9 (2010) Article DOI: 10.1016/j.bmcl.2010.09.028 BindingDB Entry DOI: 10.7270/Q2RF5V79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50252297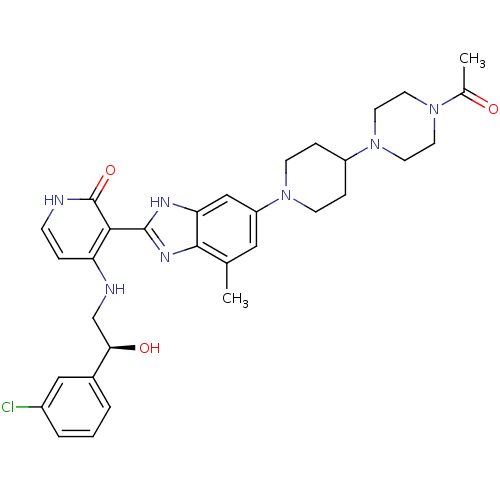 ((S)-3-(6-(4-(4-acetylpiperazin-1-yl)piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4075-80 (2008) Article DOI: 10.1016/j.bmcl.2008.05.104 BindingDB Entry DOI: 10.7270/Q2V40V03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50280456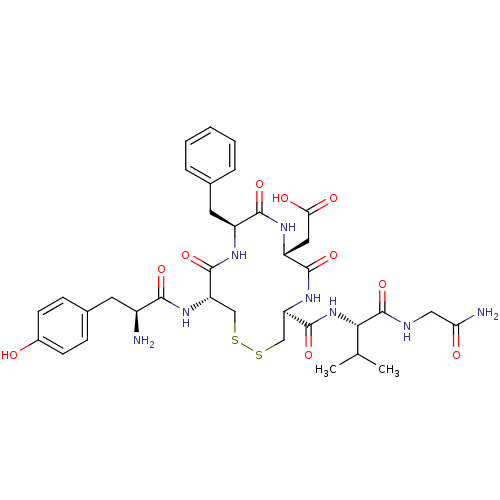 (CHEMBL153466 | {(4S,7R,10S,13R)-13-[(S)-2-Amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against delta receptor with [3H]-[p-Cl-Phe4]-DPDPE | Bioorg Med Chem Lett 2: 547-552 (1992) Article DOI: 10.1016/S0960-894X(01)81195-1 BindingDB Entry DOI: 10.7270/Q23R0STD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50043717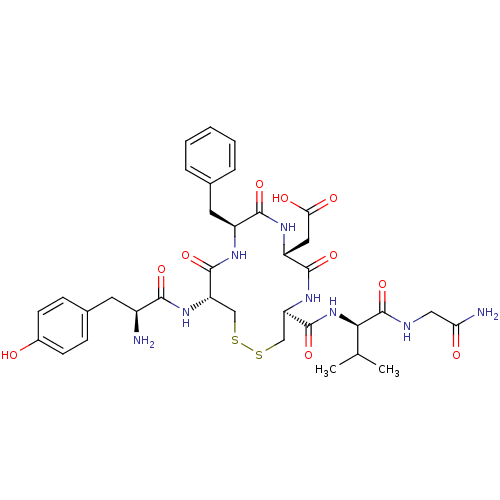 (CHEMBL289006 | {(4S,7R,10S,13R)-13-[(S)-2-Amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of [3H]-[p-Cl-Phe-]DPDE binding to rat brain homogenate delta-opioid receptor | J Med Chem 37: 141-5 (1994) BindingDB Entry DOI: 10.7270/Q2F47N7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000806 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]- DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50544198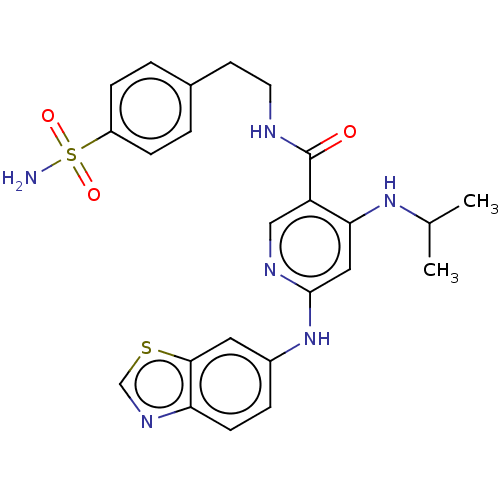 (CHEMBL4636136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Biocon Bristol Myers Squibb Research Center Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 11: 1402-1409 (2020) Article DOI: 10.1021/acsmedchemlett.0c00082 BindingDB Entry DOI: 10.7270/Q2542S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50252143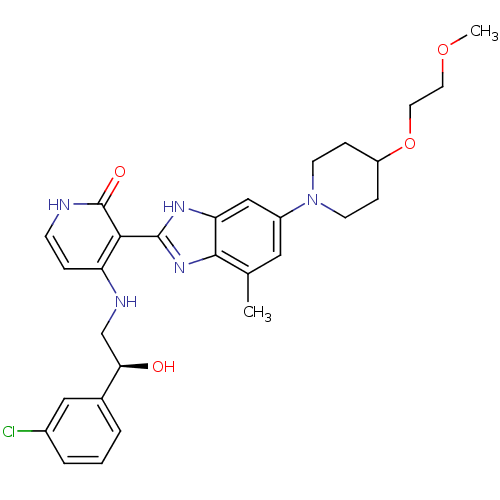 ((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem Lett 18: 4075-80 (2008) Article DOI: 10.1016/j.bmcl.2008.05.104 BindingDB Entry DOI: 10.7270/Q2V40V03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50056543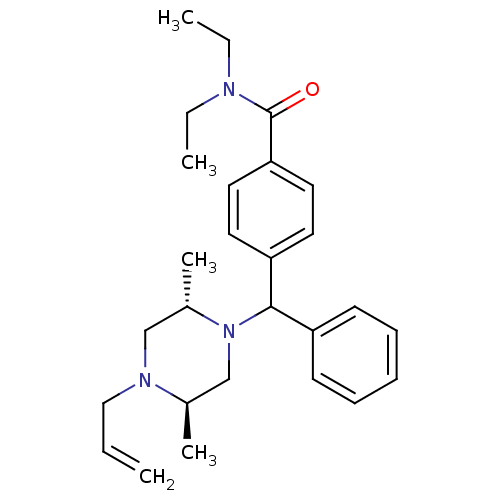 (4-[((1R,2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of radioligand [3H]DADLE binding to rat brain Opioid receptor delta 1 | J Med Chem 40: 695-704 (1997) Article DOI: 10.1021/jm960319n BindingDB Entry DOI: 10.7270/Q2F190DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50393257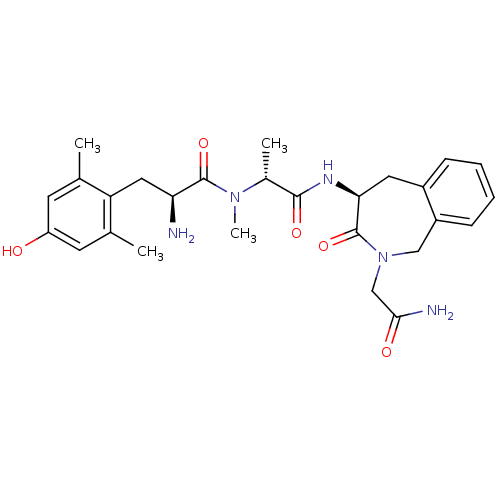 (CHEMBL2151735) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from DOR in rat brain homogenates by liquid scintillation counting | J Med Chem 54: 7848-59 (2011) Article DOI: 10.1021/jm200894e BindingDB Entry DOI: 10.7270/Q25X2B1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM31772 (1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01170 BindingDB Entry DOI: 10.7270/Q2DZ0D8J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50586268 (CHEMBL5079148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00089 BindingDB Entry DOI: 10.7270/Q2RJ4PCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 10428 total ) | Next | Last >> |